Magnesium chloride hexahidrat
ADVANTAGES OF MAGNESIUM CHLORIDE IN RELATION TO OTHER MEANS OF DE-ICING OF ROADS
1. Characteristics of magnesium chloride as a means of de-icing of roads is based not only on speed defrosting but on quantity of water resulting from the de-icing per unit of used means. The graph shows the characteristics of different means for de-icing (MgCl2, CaCl2 i NaCl).
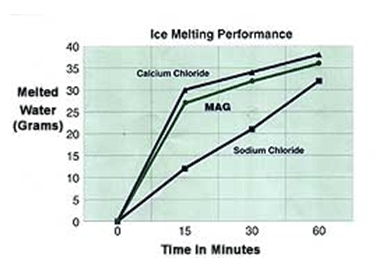
These conclusions have been based on examination of the Swedish National Institute of Roads and Transport uniform sprayings of 10 g of each means of unfreeze the frozen surface at 21°F (-6°C). As can be seen MgCl2 shows much better performance compared to CaCl2 and NaCl.
2. ACTION OF DIFFERENT TEMPERATURES
Means of temperature (in theory) °C / temperature (in practice) °C
Calcium
hliorid -50 -31
Magnesium chloride -33 -25
Rock salt (NaCl) -21 -6
Urea
-11 -11.1
Potassium chloride -11.1 -3.8
Calcium Magnesium Acetate -19.86
-3.88
* Data from literature and based on data obtained from scientific
research.
3. MAGNESIUM CHLORIDE CHLORIDE CONTAINING LESS POLLUTION AFFECTING ENVIRONMENT
Magnesium chloride contains less chloride of potassium chloride, sodium chloride and calcium chloride, so that gives an advantage when used in relation to other means of thawing.
4. USE
Magnesium chloride, except for winter
maintenance of roads, is used in various industries. An essential element in
nutrition to plants and animals.
It is used to produce a mixture of animal
nutrition and feeding of plants.
Its application is in textile, oil and
timber industries, as well as wastewater treatment.
Packed in bags of 25 kg or 1000 kg.
