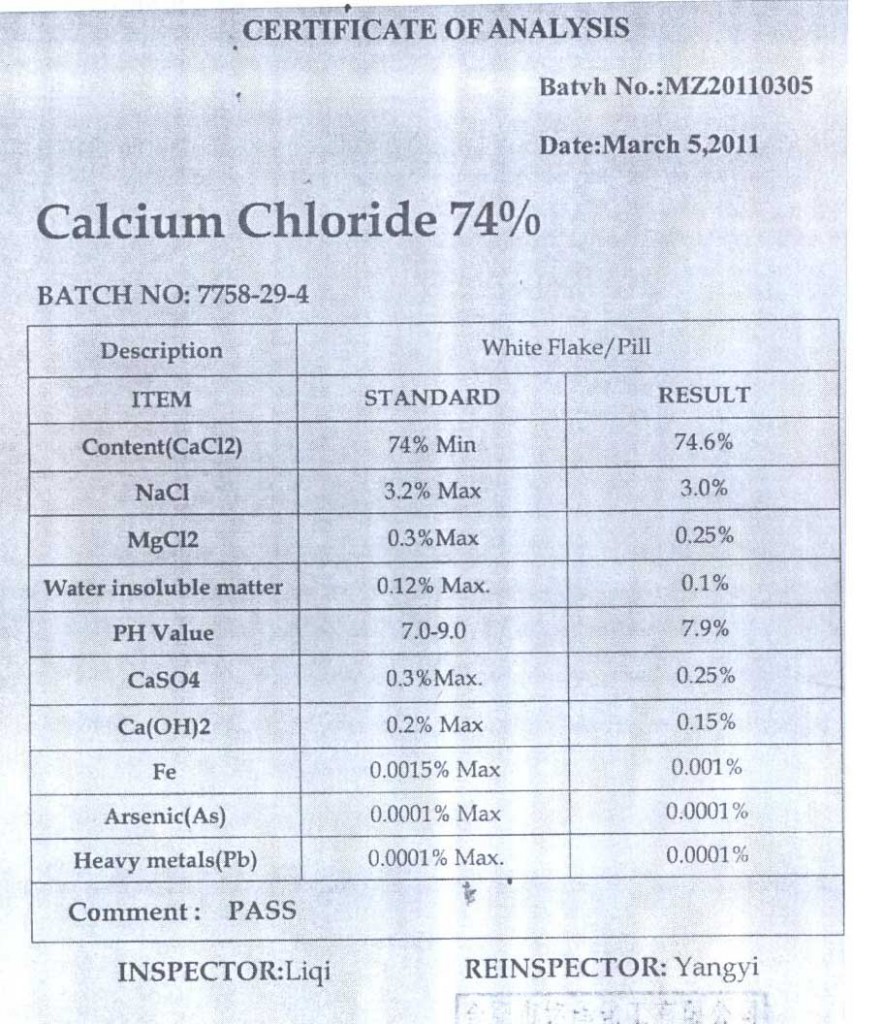
CALCIUM CHLORIDE (CALCIUM CHLORIDE 74%)
Calcium chloride (CaCl2) is the inorganic compound of calcium and chlorine. In the form of a salt which is solid at room temperature and is very hygroscopic. It is easily soluble in water. It is obtained directly from ore.
Calcium chloride is used:
As a a means of preventing ice formation on the road
Calcium chloride helps in decreasing freezing point of water. This particular feature is used for deicing roads in winter. While sodium chloride (commonly used for sprinkling the roads in winter time) takes about 19 minutes to start melting ice, calcium chloride works almost instantly. Water that contains about 30% calcium chloride freezes until on temperature of -67 degrees Celsius. Calcium chloride melts ice faster than any other chemical compounds. In extremely cold conditions can be used in powder form. Requires fewer applications than in NaCl, which reduces salt consumption by about 40% as well as involvement labor force and machinery.
Addition of calcium chloride was found in the application:
- medicine as a means to compensate for calcium in the organism
- for dehydration of gases
- for getting variety of calcium compounds
- to remove traces of water from organic substances
- in the food industry as an additive